Aluminum corroding refers to the process by which aluminum undergoes deterioration or damage due to the reaction with its surroundings. This corrosion occurs when the protective oxide layer on the surface of aluminum is compromised, allowing external elements, such as moisture and certain chemicals, to interact with the metal.
The intriguing question sparks curiosity about the potential interactions between stainless steel and aluminum. Will Stainless Steel Corrode Aluminum? It explores the compatibility of these two metals, raising awareness about the possibility of corrosion when they come into contact.
Stainless steel corroding aluminum involves the risk of galvanic corrosion, a phenomenon arising from the contact between dissimilar metals. In this process, stainless steel, known for its corrosion resistance, may corrode aluminum when exposed to certain environmental conditions.
How Does Aluminum Corrode?
Corrosion of aluminum is a natural process where the metal deteriorates due to environmental factors. Aluminum forms a protective oxide layer on its surface, shielding it from corrosion. When this layer is compromised, exposure to moisture, air, or certain chemicals can trigger corrosion.
The metal undergoes various forms of degradation, including rusting and pitting, diminishing its structural integrity over time. The mechanisms behind aluminum corrosion are vital for comprehending how this lightweight and versatile metal interacts with its surroundings, setting the stage for exploring its compatibility with other metals like stainless steel.
Will Stainless Steel Corrode Aluminum?
The compatibility between stainless steel and aluminum is a critical consideration, especially in applications where these metals come into close contact. The question, Will stainless steel corrode aluminum? arises from the potential risk of galvanic corrosion, a process driven by the electrochemical interaction between dissimilar metals.
Stainless steel, lauded for its corrosion resistance, may inadvertently corrode aluminum when exposed to specific environmental conditions. This compatibility issue holds significance in diverse settings, from household items to industrial equipment, prompting the need for a deeper understanding of the interplay between stainless steel and aluminum.
A Threat to Aluminum Though Galvanic Corrosion
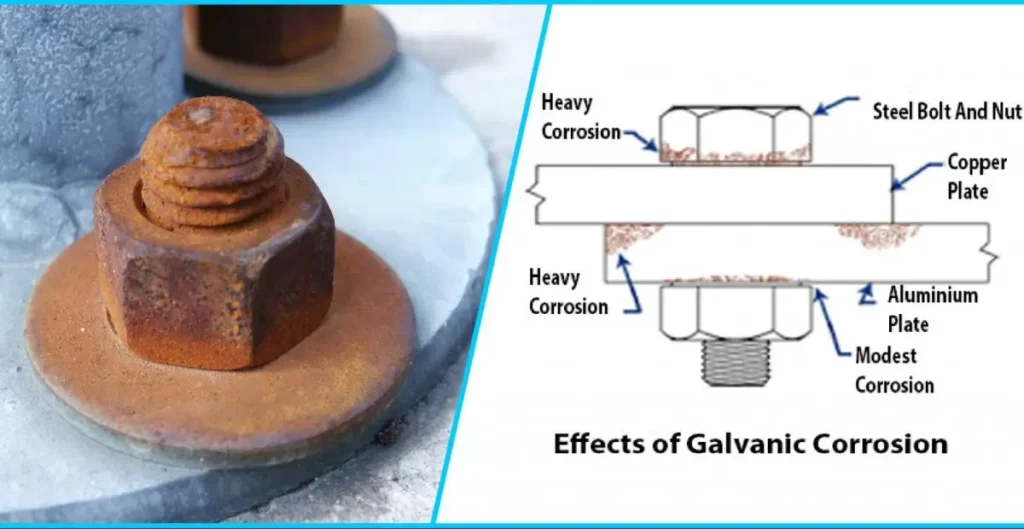
Galvanic corrosion poses a substantial threat to aluminum when paired with stainless steel. This form of corrosion occurs due to the differing electrochemical potentials of the metals. These metals are in contact, electrons flow from one to the other, accelerating the corrosion of the more reactive metal, in this case, aluminum.
The threat of galvanic corrosion becomes particularly pronounced in environments where moisture or other corrosive elements are present. Exploring the nuances of galvanic corrosion sheds light on the potential risks and underscores the importance of careful material selection and preventive measures to safeguard aluminum from the corrosive impact of stainless steel.
Identifying Environmental Triggers for Stainless Steel and Aluminum Interaction
The environmental triggers that lead to the interaction between stainless steel and aluminum is crucial in preventing corrosion. One primary trigger is exposure to moisture, as water can facilitate the electrochemical reactions between the metals. The presence of certain chemicals or pollutants in the environment can accelerate the corrosion process.
The combination of moisture and chemical exposure weakens the protective oxide layer on aluminum, making it more susceptible to corrosion when in contact with stainless steel. Controlling the environmental conditions, such as humidity and the presence of corrosive substances, becomes essential in preserving the integrity of both metals and preventing the undesirable interaction.
Risks of Stainless Steel Turning Green on Aluminum
In real-world applications, the risks associated with stainless steel turning green on aluminum are prevalent, especially in outdoor settings. This green discoloration often results from galvanic corrosion, where the stainless steel corrodes the aluminum. For instance, in architectural structures with mixed metal components, such as stainless steel and aluminum railings or facades.
Prolonged exposure to environmental factors like rain and pollutants can lead to the development of a greenish patina on the aluminum surfaces. This not only compromises the aesthetics but also raises concerns about the structural integrity of the affected components.
Corrosion Between Stainless Steel and Aluminum
Examining case studies provides valuable insights into instances of corrosion between stainless steel and aluminum, shedding light on the real-world consequences of their interaction. In marine environments, for example, where both metals are commonly used in boat construction, galvanic corrosion is a prevalent issue.
The tarnishing of a stainless steel necklace is hastened by the close proximity of stainless steel and aluminum in saltwater, accelerating degradation. Case studies emphasize the need for environmental consideration and preventive measures against corrosion risks.
Preventing Stainless Steel from Turning Aluminum Green
When it comes to preventing stainless steel from turning aluminum green, implementing effective mitigation strategies is paramount. One key approach is to create a barrier between the two metals to impede direct contact. This can be achieved by using insulating materials or coatings that act as a protective layer, inhibiting the galvanic corrosion process.
Applying corrosion inhibitors or protective films on the aluminum surface helps maintain the integrity of the metal. Regular inspections and maintenance routines are crucial to identify any signs of corrosion early on, allowing for prompt intervention before significant damage occurs.
Choosing the Right Metals for Specific Environments
Selecting the right metals for specific environments is a fundamental aspect of preventing stainless steel from turning aluminum green. The environmental conditions, such as humidity, temperature, and exposure to corrosive substances, is crucial in making informed choices.
In marine or coastal settings, where the risk of corrosion is high, opting for stainless steel grades with enhanced corrosion resistance becomes essential. Choosing aluminum alloys designed for specific applications minimizes the likelihood of corrosion.
FAQs
Can stainless steel turn aluminum green?
Stainless steel can induce green discoloration on aluminum through galvanic corrosion, a process where the dissimilar metals interact, causing deterioration and potentially turning the aluminum green.
What environmental factors contribute to stainless steel corroding aluminum?
Moisture and certain chemicals in the environment can compromise the protective oxide layer on aluminum, facilitating the galvanic corrosion process when in contact with stainless steel.
How can one prevent stainless steel from corroding aluminum?
To prevent stainless steel from turning aluminum green, careful material selection, coatings, and insulation methods are essential, and minimizing the risk of galvanic corrosion.
Conclusion
The potential for stainless steel to corrode aluminum is key to preserving the integrity of these metals. We’ve explored the risks involved, particularly with the threat of galvanic corrosion, and how it can impact various applications. By being mindful of the environmental conditions and implementing effective mitigation strategies.
We can ensure that stainless steel and aluminum coexist harmoniously, without the undesirable outcome of stainless steel turning green on aluminum surfaces. This awareness enables us to make informed choices in material selection, promoting longevity and reliability in diverse settings.

